HAMA and Interference Blocker
These sample or antibody dilution buffers are used to reduce interference effects such as HAMA, rheumatoid factors, non-specific binding, cross-reactivities and matrix effects in immunoassays. False positive and false negative results can be avoided by using these buffers, thus increasing the reliability of the assays.
Stabilizers
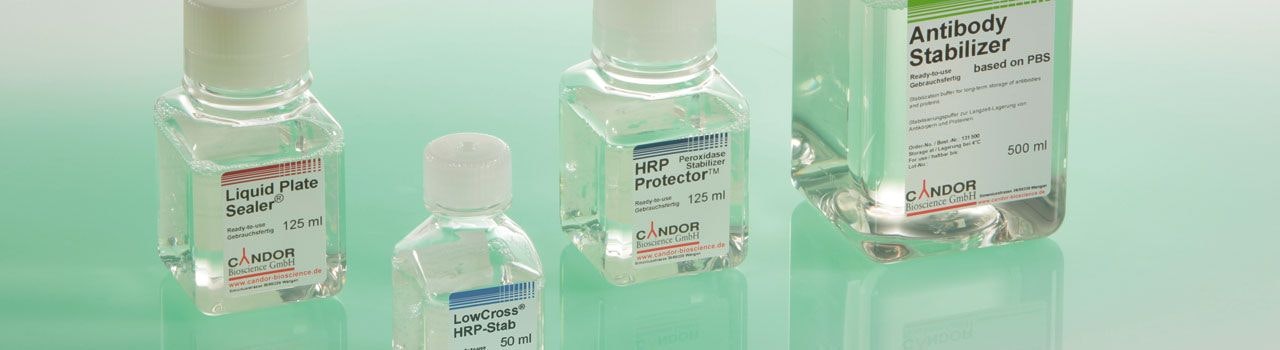
Immunoassay stabilizers are needed to preserve the native conformation of proteins and protect them from losing their functionality. CANDOR stabilizers enable long-term storage of antibodies, conjugates (eg HRP-labelled or AP-labelled) and antigens in solution or coated on microtiter plates and other surfaces. The product range extends from Antibody Stabilizer to HRP-Protector™ , AP-Protector® , LowCross® HRP-Stab to Liquid Plate Sealer®.
To always get reliable results in immunoassays it is absolutely necessary to also stabilize standards for calibration curves, coated plates and antibodies during storage. CANDOR's stabilizers can be used to maintain the activity of all biological components of an immunoassay . CANDOR stabilizers are regularly used for the production of commercial ELISA kits with a shelf life of 2 or more years.
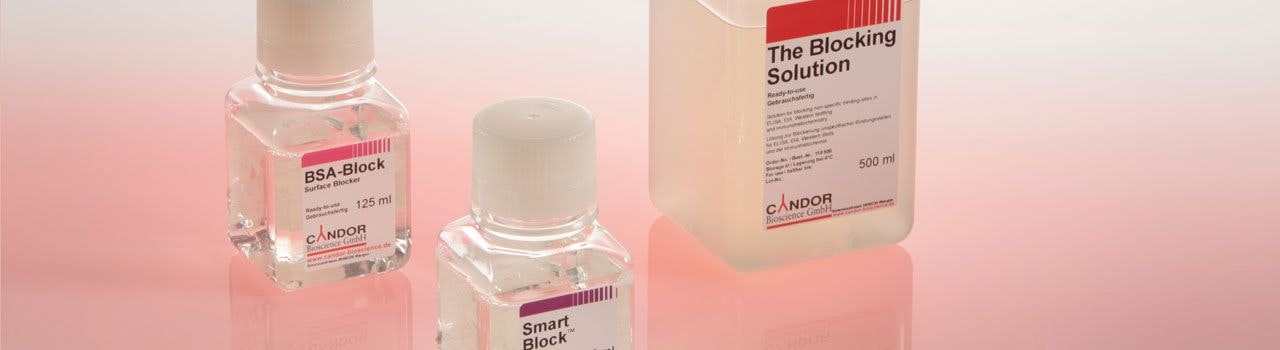
Surface Blocker
The surfaces of ELISA plates or Western Blot membranes are optimized for efficient binding of proteins and antibodies . This enables optimal high-density assays on capture antibodies or capture antigens on the surface. As a result of this surface optimization, many other proteins, peptides or other molecules from the sample can also bind to the surface. Without blocking the surface, assay antibodies, tracers, analytes, or other sample components would bind to the surface. This can lead to incorrect results or high background signals. It must therefore be prevented in order to obtain reliable assays. The blocking is necessary in order to saturate free binding sites on the respective surface. This saturation occurs by covering the surface with a dense, uninterrupted layer of molecules. CANDOR offers four different blockers with different composition and blocking properties. BSA-Block is a classic BSA-based blocker for many applications. SmartBlock™ is a very efficient peptide-based blocker. The Blocking Solution is a high-quality casein-based blocker for routine applications as well as for challenging immunoassays . PlateBlock™ is an ELISA plate blocker for use in serological assays (antigen down format).
Buffer
CANDOR Buffer Solutions are all ready-to-use solutions or 10x concentrates that allow easy and convenient work with immunoassays . All solutions are manufactured in-house under current DIN EN ISO 9001 standards . These include, for example, in-process controls, release controls and the traceability of all components of the solution. This means that all products can be used directly in regulated areas such as GLP laboratories (Good Laboratory Practice) or for the production of diagnostic kits. The constant lot to lot quality meets the highest requirements.
CANDOR packages
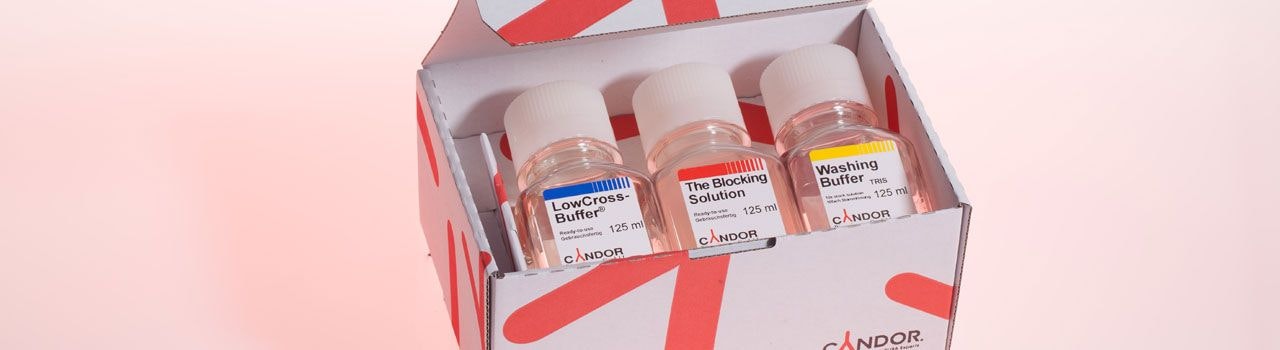
In a first test you can quickly determine which solutions are ideal for an assay. To make things easier, we offer you suitable combinations of inexpensive packages.
ReadyTector® - All-in-one detection solution for Western blots
ReadyTector® contains all the ingredients needed for rapid, one-step immunodetection.
Each user only adds his specific primary antibody. All-in-one means that everything is included in one solution and everything is done in one step. Blocking and binding of the primary and secondary antibodies occur simultaneously. After that, only the special ReadyTector® wash buffer is used for washing. The already used ReadyTector® solution with the valuable primary antibody can be reused up to 5 times.
Despite the rapid one-step incubation, ReadyTector® reduces background, allowing for clear and sharp bands. The results can thus be published directly.
ReadyTector® for Western blotting – easy, quick and clear
- easy – simple application
- quick – quick execution and quick result
- clear - no background
ReadyTector® is available as Anti-Mouse HRP and Anti-Rabbit HRP.
The ReadyTector® Kit contains the all-in-one solution and also the ReadyTector® 10x wash buffer in bottle sizes of 40 ml, 120 ml and 500 ml.
Important questions and answers about ReadyTector® can be found here.
For more information on ReadyTector®, visit www.readytector.com.

Animal free/protein free
Especially for veterinary diagnostic assays, we also offer some of our stabilizers and assay diluents as protein-free or animal-free variants.
Products marked as protein-free by CANDOR are manufactured without the use of raw materials containing protein. No proteinaceous material is present during the manufacturing process. CANDOR products designated as protein-free can also be animal-free.
Products marked animal-free by CANDOR are manufactured without the use of materials of animal or human origin. We only use raw materials and primary packaging that have been declared animal-free by the respective supplier. No material of animal or human origin is present during the manufacturing process.